COVID-19 Testing
Prevent an outbreak that would disrupt your business and give your staff peace of mind.
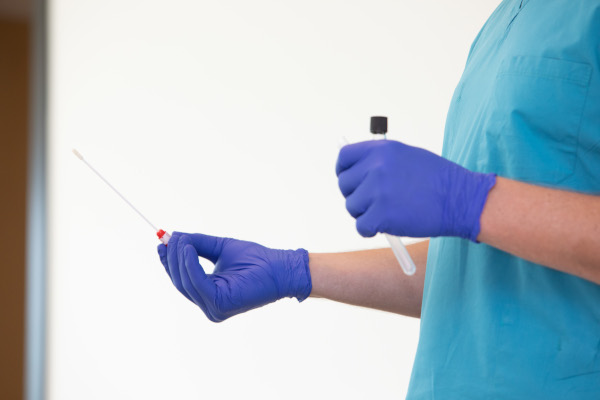
Convenient On-site Testing
Our medical staff comes to your business to perform testing, eliminating scheduling and travel issues. COVID-19 diagnostic tests can be facilitated to screen your employees for infection.
Additionally, COVID-19 antibody tests can be administered to determine if any of your employees have previously been exposed to the virus that causes COVID-19, and if so, whether or not they have developed antibodies.
Planning and coordination for testing is a seamless process; our staff makes it easy to test any size group of people. Testing can be scheduled on a one-time or recurring basis.
Test samples are collected and couriered to our lab partner by our trained personnel. COVID-19 diagnostic and antibody test results are available within 24-48 hours of receipt of the samples by our lab partner.

Self-Testing Kits
Self-testing kits are a diagnostic test for COVID-19. This type of test uses a saliva sample to check whether your employee has an active COVID-19 infection and can spread it to others. It is not a COVID-19 antibody test, which determines whether your employee has had COVID-19 in the past.
Each kit contains a sealable vessel for your employee to fill with a small amount of saliva. Once the sample is self-collected, it should be sent directly to us (FedEx postage is included). Results are emailed to the participant within 24-48 hours of receipt of the sample by our lab partner.
Individual self-testing kits for COVID-19 diagnostics can be purchased on an as needed basis. Many businesses opt to purchase a bulk number of these kits to keep on-hand for convenience.
A Detailed Look at Diagnostic and Antibody Testing
COVID-19 Diagnostic Testing
Our on-site COVID-19 testing is done using a nasal mid-turbinate swab. This test is quick and relatively non-invasive (painless), although some individuals may find it uncomfortable.
Our laboratory partner, US Biotek, uses RT-PCR technology, the current preferred testing method of the Centers for Disease Control (CDC), to test nasal swab and salive samples. By identifying the RNA (genetic material) of the virus, their test specifically detects SARS-CoV-2 virus in patients’ cells. 99% of results are available within 24 hours after the lab receives a specimen.
COVID-19 Antibody Testing
US BioTek performs their COVID-19 antibody testing on a high-sensitivity chemiluminescent platform. This kind of testing detects the presence of IgM and IgG antibodies to SARS-CoV-2, indicating past or recent exposure – and potential immunity – to the virus.


